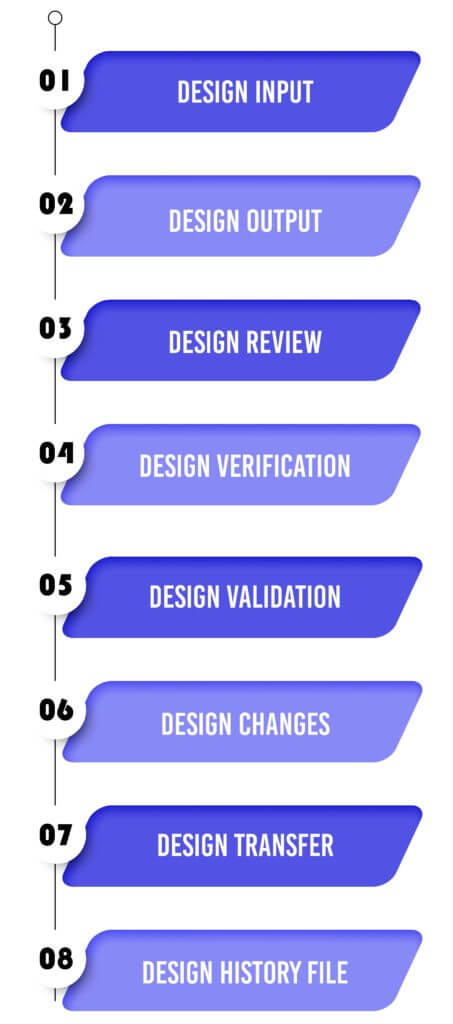
9 Tips for Addressing the Documentation Burden of the FDA's Design Control Regulation - Medical Design Briefs

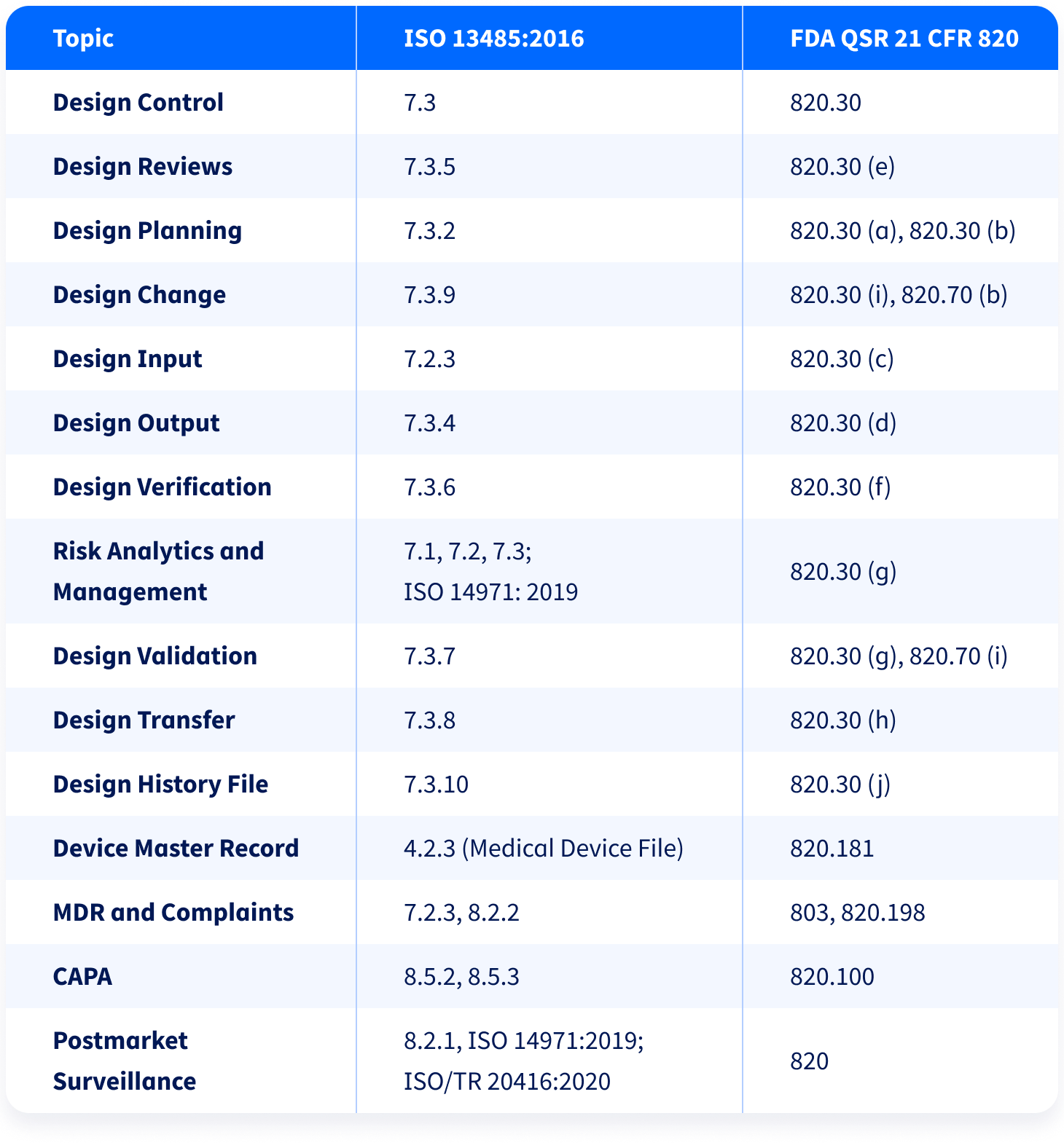
US FDA 21 CFR 820.30 (Documentation and Process for Design Controls For Medical Devices) | Operon Strategist | Isometric, Medical, Online registration
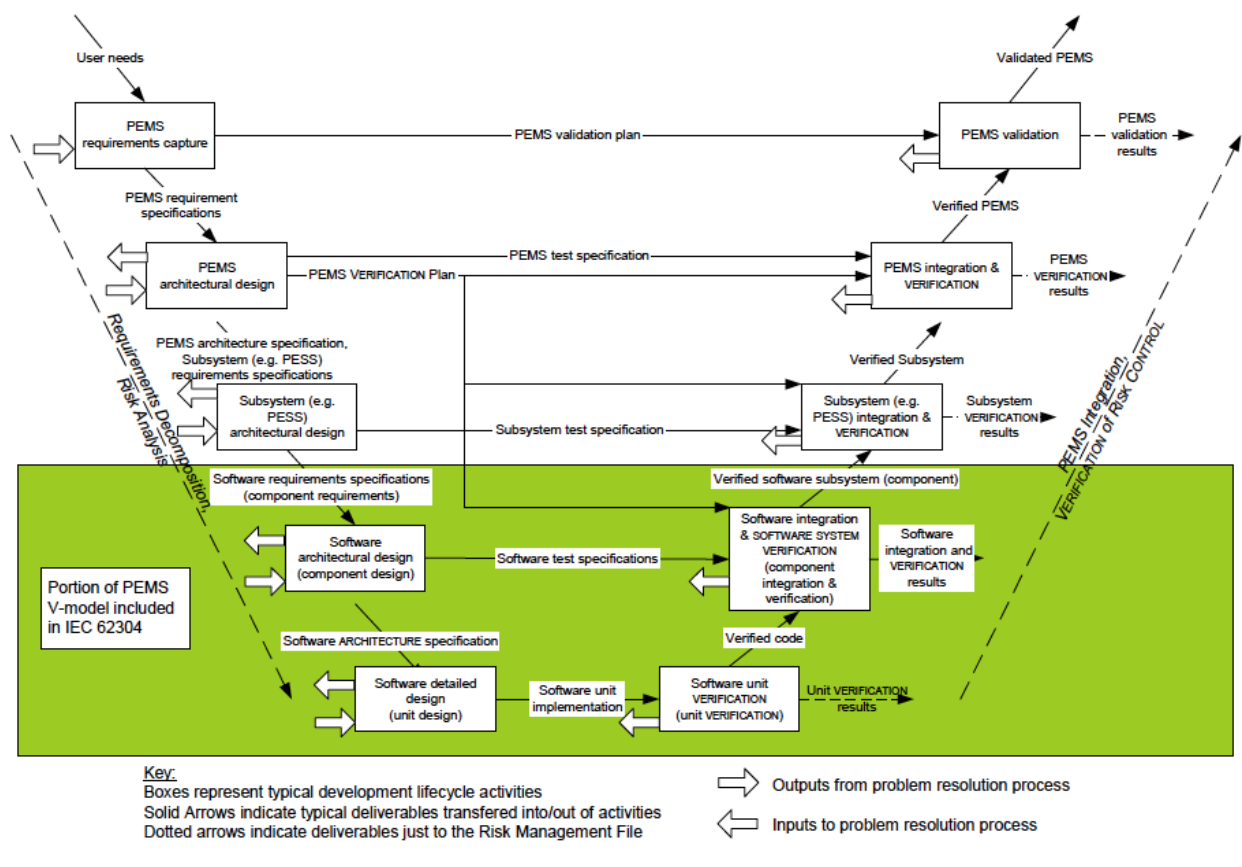
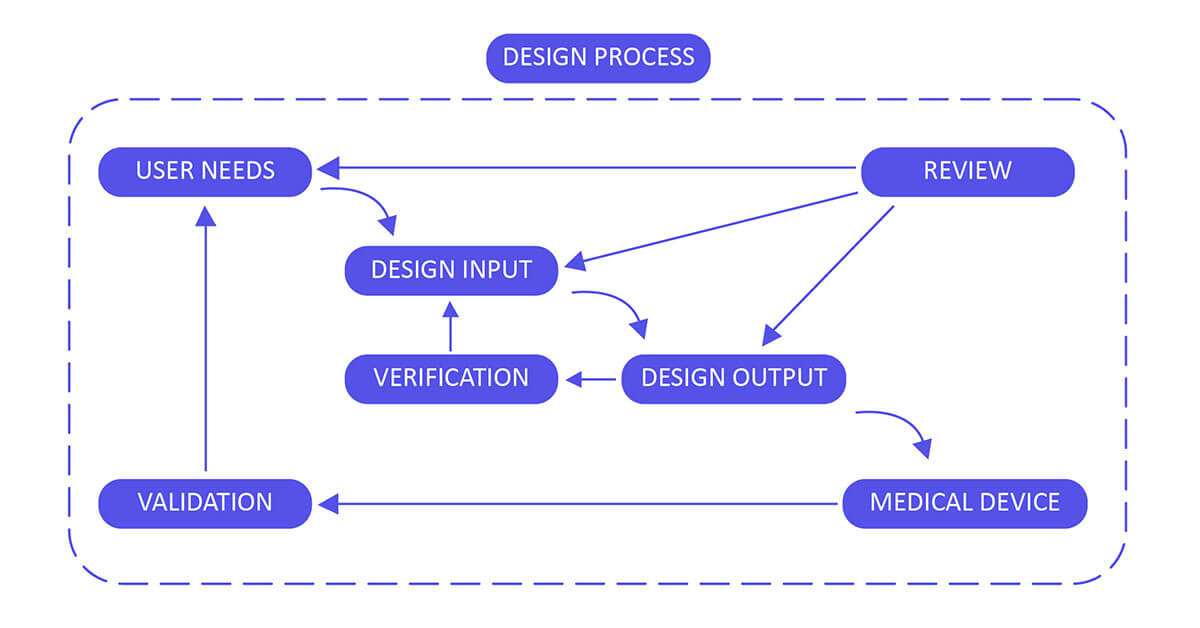
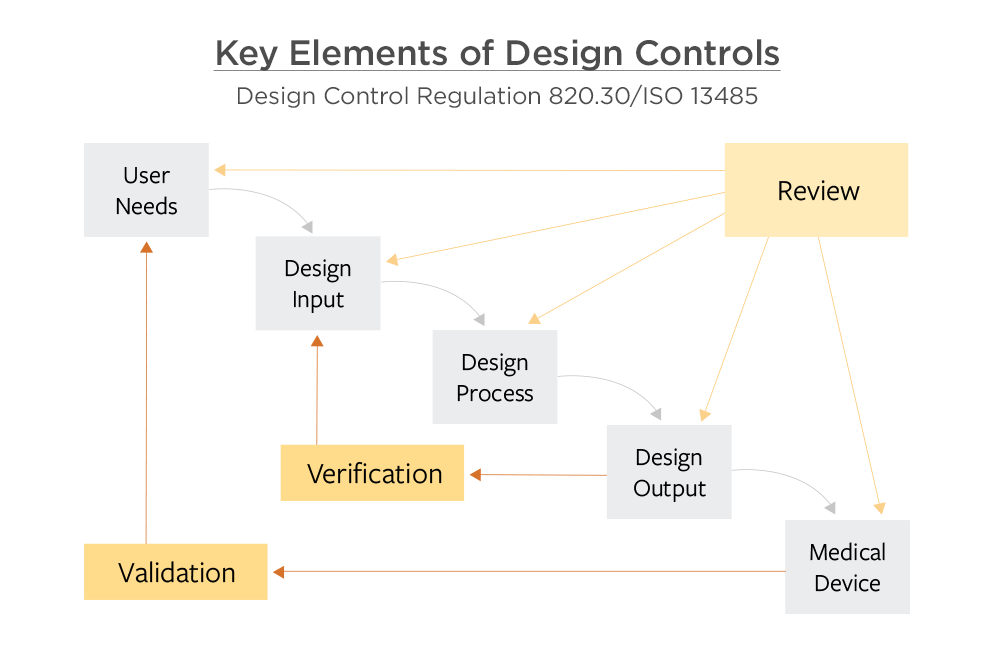
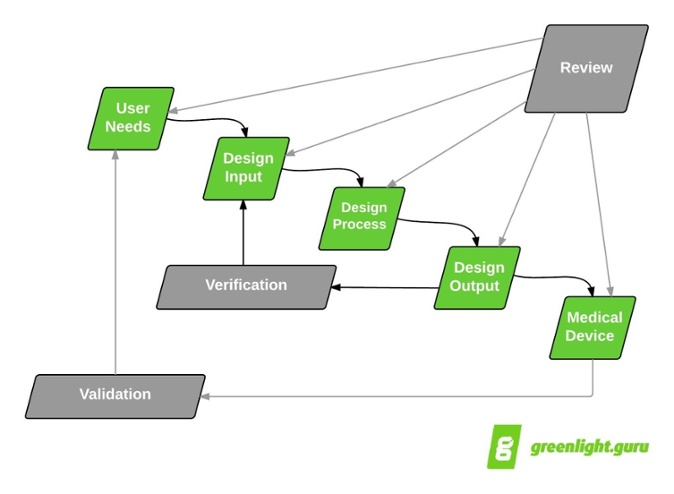
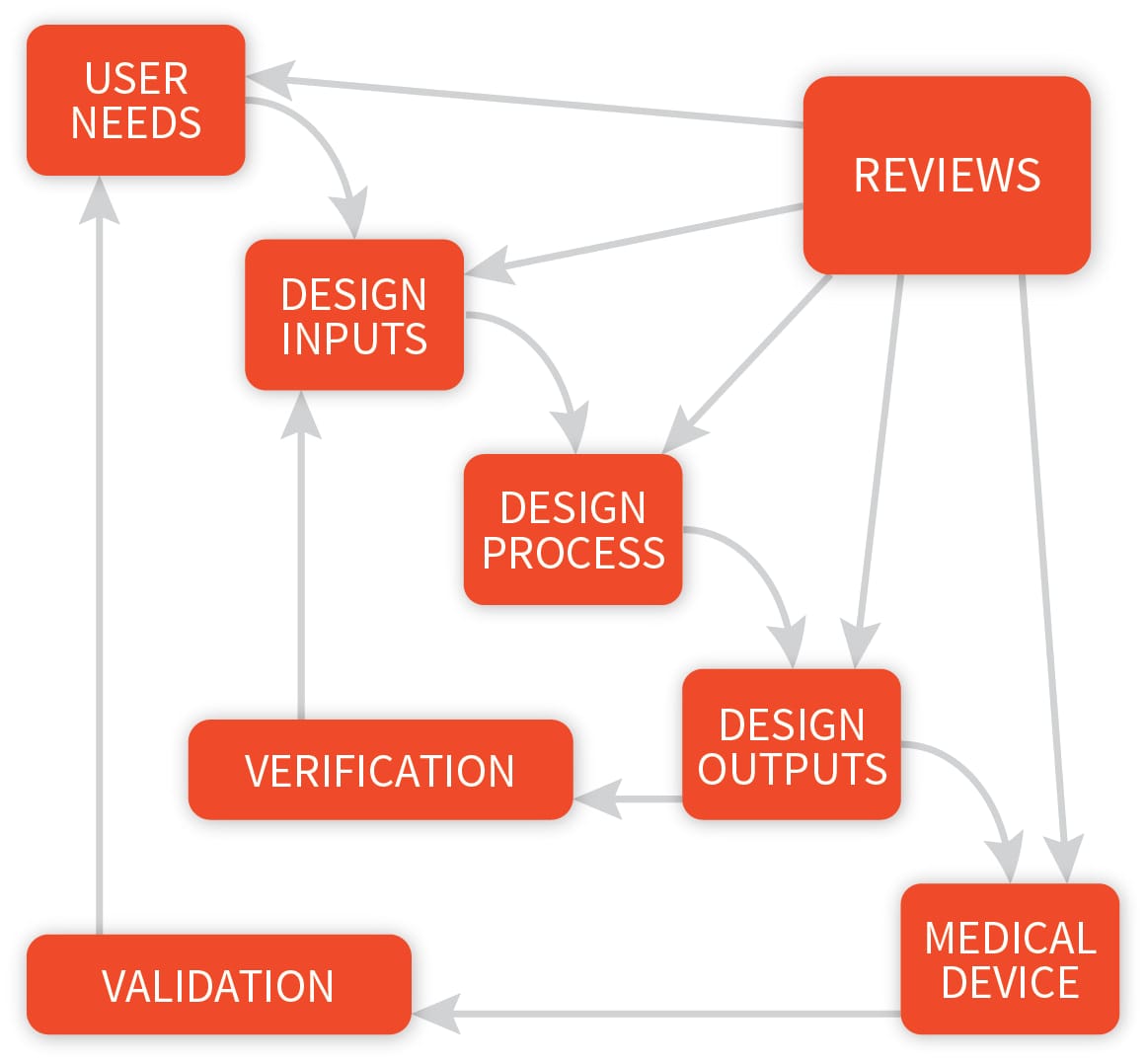
Application of design controls to design process. Design review should... | Download Scientific Diagram

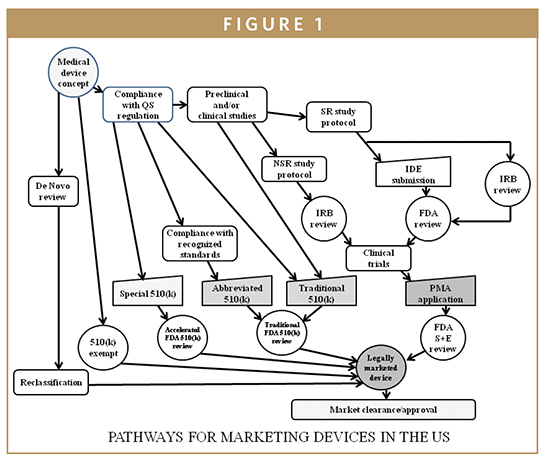
The steps in the FDA-mandated Design Control process (center section... | Download Scientific Diagram

21 CFR Part 820 - Quality System Regulation | 21 CFR 820.30 Medical Device Design Control Guidelines - YouTube

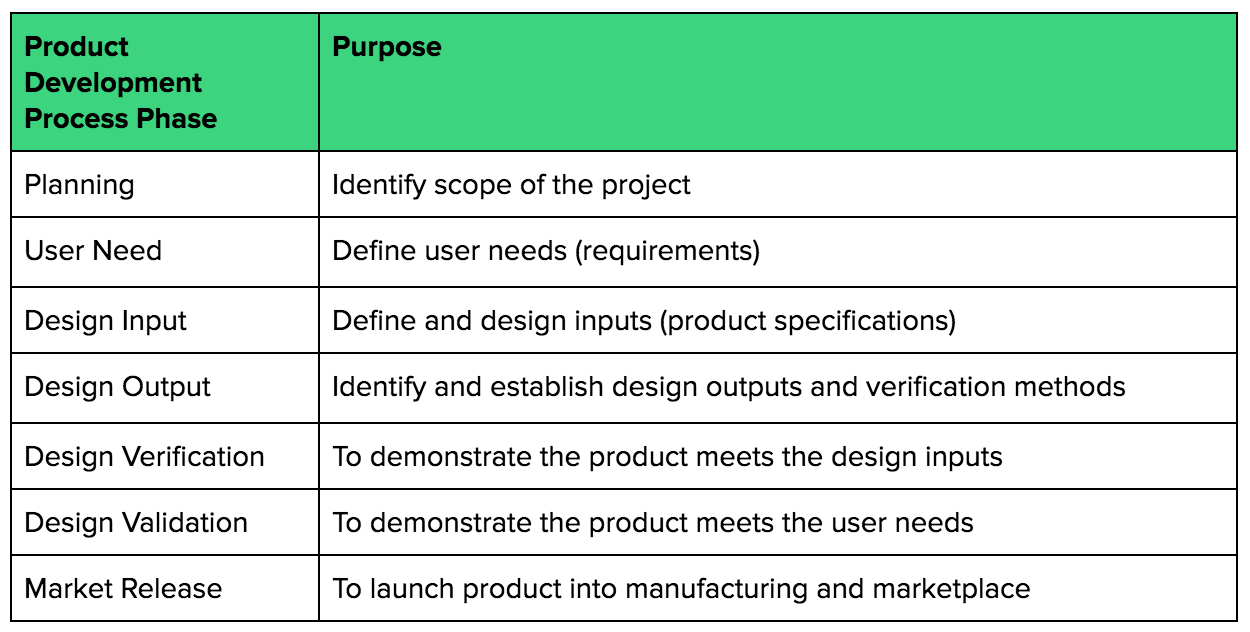
Agile Development in Regulated Environments Example: Medical Devices – Waterfall Lifecyle Model | Scaling Software Agility

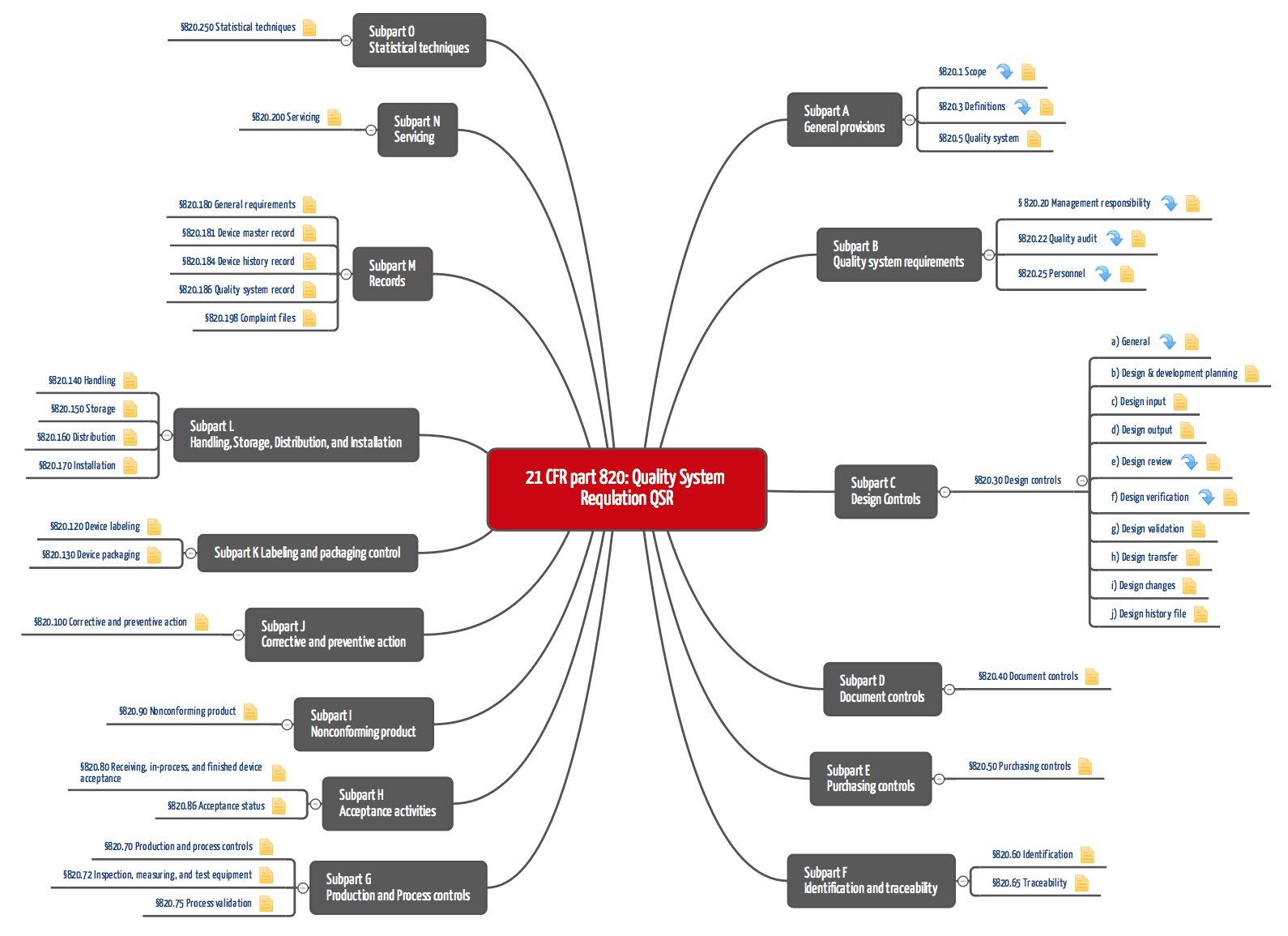
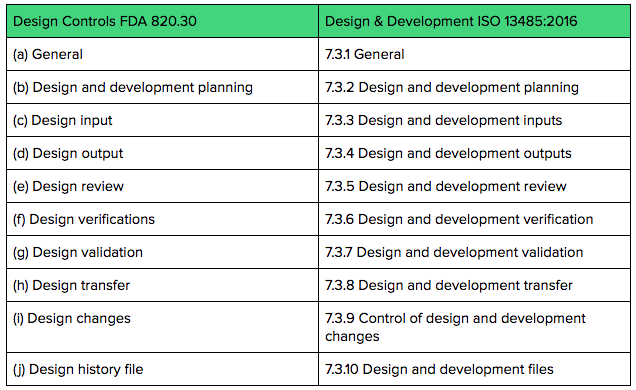
Design Controls: Building Objective Evidence and Process Architecture to Meet FDA and ISO Compliance - OMTEC 2018 | PPT